The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction between azides and alkynes to form 1,2,3-triazoles, as reported1 by Sharpless, was found to be so exquisitely regioselective and efficient at even the most mild conditions that Sharpless coined the term ‘Click Chemistry’ to describe it. The use of this method for DNA modification has been somewhat delayed by the fact that copper ions damage DNA, typically yielding strand breaks.2 As these problems have now been overcome by the use of copper(I)-stabilizing ligands (e.g., tris(benzyltriazolylmethyl)amine, TBTA3), Carell et al. and Seela et al. discovered that the CuAAC reaction can be used to functionalize alkyne-modified DNA nucleobases with extremely high efficiency.4
At Glen Research, our goal was to offer a copper-free click phosphoramidite reagent with the following properties:
From the variety of cyclooctyne-based copper-free click reagents so far described, we have chosen to offer compounds based on a dibenzo-cyclooctyne (DBCO) structure.
References
[1] C.W. Tornoe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057-3064; V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708-2711; Angew. Chem. Int. Ed. 2002, 41, 2596-2599.
[2] C. J. Burrows, J. G. Muller, Chem. Rev. 1998, 98, 1109 – 1151.
[3] T. R. Chan, R. Hilgraf, K. B. Sharpless, V. V. Fokin, Org. Lett. 2004, 6, 2853 – 2855.
[4] J. Gierlich, G. A. Burley, P. M. E. Gramlich, D. M. Hammond, T. Carell, Org. Lett. 2006, 8, 3639-3642. F. Seela, V. R. Sirivolu, Chem. Biodiversity 2006, 3, 509-514.

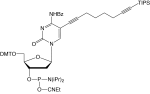
5'-Dimethoxytrityl-5-[(6-oxo-6-(dibenzo[b,f]azacyclooct-4-yn-1-yl)-capramido-N-hex-6-yl)-3-acrylimido]-2'-deoxyUridine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite