Substitution of C-5 propynyl-dC (pdC) for dC and C-5 propynyl-dU (pdU) for dT are effective strategies to enhance base pairing. Using these base substitutions, duplex stability and melting temperatures are raised by the following amounts: C-5 propynyl-C 2.8° per substitution; C-5 propynyl-U 1.7° per substitution. AP-dC (G-clamp) substitutes for dC and is another very important modified nucleoside that enhances hybridization by 7-21° per substitution depending upon the sequence and location of the AP-dC. The ability of these modified bases to enhance binding while maintaining specificity has proven useful in antisense research and in the synthesis of high affinity probes. AP-dC is also a fluorescent nucleoside and should find uses in DNA structural research.
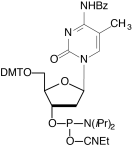
C-5 methyl pyrimidine nucleosides are known to stabilize duplexes relative to the non-methylated bases. Therefore, enhanced binding can be achieved using 5-methyl-dC in place of dC, duplex melting temperature being increased by 1.3°. Ac-5-Me-dC-CE Phosphoramidite is fully compatible with AMA deprotection and none of the N4-Me transamination mutant is observed on deprotection.
The simplest approach to the design of high affinity primers and probes is to substitute A sites with 2-amino-A, since the 2-amino-A-T base pair is equivalent in strength to the G-T base pair. 2-Amino-A also destabilizes A-G wobble mismatches, thus increasing specificity. In 1998, we introduced a 2-amino-dA monomer which exhibits fast and effective deprotection in ammonium hydroxide and it is stabilized to depurination during synthesis. We now recommend the use of 0.5 M CSO in anhydrous acetonitrile (40-4632-xx) for best results with multiple additions of 2-amino-dA. This is because the bis formamidine protected 2-amino-dA leads to significant strand scission when standard iodine oxidation is used during synthesis. For this reason, we have also added Pac-2-Amino-dA, a monomer with optimized protection to meet the following criteria: stable during oligonucleotide synthesis, oxidation, and detritylation; labile towards common deprotection conditions (NH3, AMA, MeNH2); and the nucleobase protecting groups are cleaved under fairly mild conditions.
Sequences with high GC content may contain mismatches and still hybridize because of the high stability of the G-C base pair. The N4-ethyl analogue of dC (N4-Et-dC) hybridizes specifically to natural dG but the stability of the base pair is reduced to about the level of an AT base pair.
Coupling N6-Me-dA (10-1003) and N4-Et-dC (10-1068) with 1H-tetrazole leads to a trace of branching at the secondary amine positions, while DCI leads to around 15% branching. In collaboration with Berry and Associates, the acetyl protected monomers were prepared. Acetyl protection was chosen since it would block branching reactions. Oligonucleotides synthesized using these monomers proved to be compatible with all popular deprotection strategies from UltraMild to UltraFast. When the acetyl protected monomers were compared with the unprotected monomers using DCI as activator, branching was reduced from 15% to zero.
dW is a C-nucleoside that acts as a strong adenine base paring analog. In addition to the typical two hydrogen bonds found between T and A, dW can also interact with A via van der Waals forces. The result is a dW–A interaction that approaches the strength of a C–G base pair while also exhibiting enhanced base-pairing fidelity. dW can be used in place of T as a single substitution or a complete replacement for oligonucleotide hybridization applications.

3-Methyl-2-pivaloyloxy-5-[2'-deoxy-3'-O-(2-cyanoethyl-N,N-diisopropylamino)-phosphino-5'-O-(dimethoxytrityl)-beta-D-ribofuranos-1'-yl]-6-(triisopropylsilylethynyl)-pyridine



1-[5'-O-(4,4'-Dimethoxytrityl)-β-D-2'-deoxyribofuranosyl]-9-(2-trifluoroacetamidoethoxy)-1,3-diaza-2-oxophenoxazine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]phosphoramidite