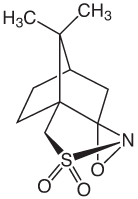
Glen Research recently began offering phosphoramidites for the synthesis of phosphonoacetate (PACE) modified oligonucleotides.1 These PACE modified oligonucleotides offer improved cellular uptake of oligonucleotides in cell culture, enhanced nuclease resistance, and the ability to stimulate RNase activity.2-4 Synthesis of PACE modified oligonucleotides requires a very mild oxidation reagent to maintain the desired PACE modification and reduce the formation of phosphodiester linkages. For the synthesis of fully PACE modified oligonucleotides, the recommended oxidation reagent is 0.10M (1S)-(+)-(10-camphorsulfonyl)oxaziridine (CSO) in anhydrous acetonitrile.2 This non-aqueous oxidation reagent prevents the formation of phosphodiester linkages during the oligonucleotide synthesis and is used in place of the standard 0.02M Iodine oxidation solution. In the past, we have recommended commercial sources of CSO but found that they are expensive and the solutions required filtering prior to use on a standard synthesizer. To support the development PACE modified oligonucleotides, we are now offering 0.10M(1S)-(+)-(10-camphorsulfonyl)oxaziridine in anhydrous acetonitrile as the recommended oxidation solution for PACE modified oligonucleotides.