Researchers have continued to explore the potential uses of C-5 propynyl pyrimidine derivatives of oligonucleotides. Caltech investigators1 have examined the effect of C-5 propynyl-dU on triple helix formation. Groups from Gilead Sciences and The Agouron Institute2 have measured the specific effect of a series of C-5 propyne oligonucleotides on HIV mRNA targets
Froehler and coworkers3 have already examined the effect of C-5 propynyl pyrimidine modifications in the behavior of 2'-O-allyl-RNA. Since our main focus in RNA monomer supply is on 2'-OMe-RNA, we believe that the enhanced binding of C-5 propynyl groups in 2'-OMe-RNA would be beneficial in the preparation and use of antisense RNA probes. We, therefore, have prepared C5-propynyl-2'-OMe-C and C5-propynyl-2'-OMe-U-CE Phosphoramidites. Structures and Ordering Information are shown on the Back Page. It should be cautioned that the U analogue is quite insoluble in acetonitrile and we recommend the use of THF as solvent and/or manual coupling for this monomer.
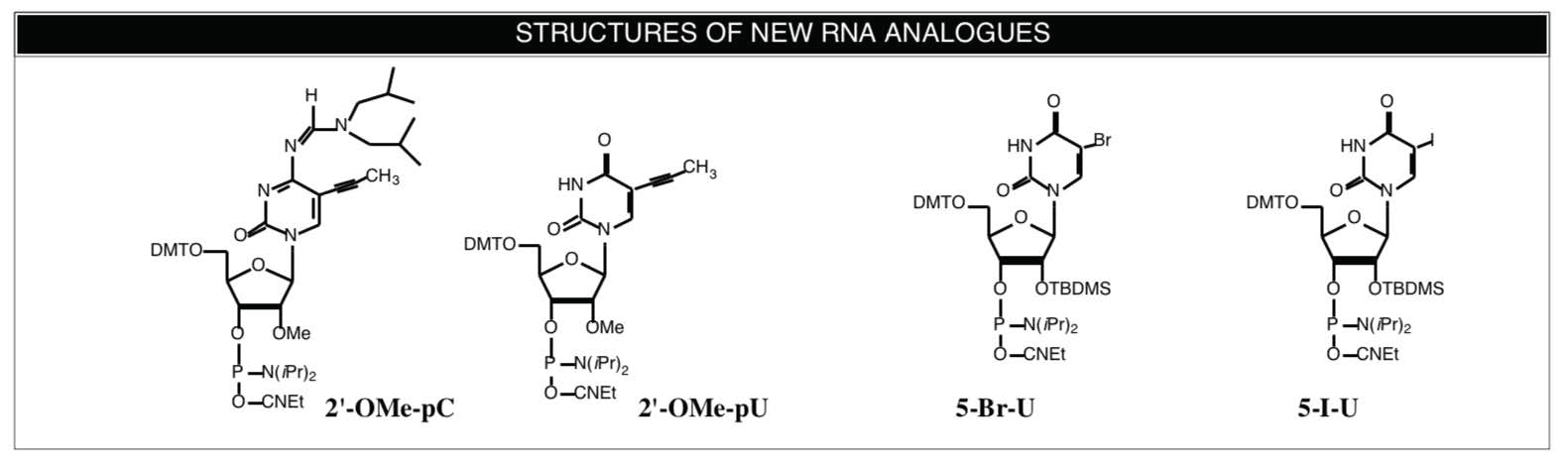
Interest in crosslinking experiments has not been restricted to DNA protein interactions and we have been asked to provide halogenated RNA monomers. The two Uridine derivatives 5-Br-U and 5-I-U4 are now available from Glen Research.
(1) N. Colocci and P.B. Dervan, J. Amer. Chem. Soc., 1994, 116, 785-786.
(2) S.D. Fenster, R.W. Wagner, B.C. Froehler, and D.J. Chin, Biochemistry, 1994, 33, 8391-8398.
(3) B.C. Froehler, R.J. Jones, X.D. Cao, and T.J. Terhorst, Tetrahedron Lett., 1993, 34, 1003-1006.
(4) K. Shah, H. Wu, and T.M. Rana, Bioconjugate Chemistry, 1994, 5, 508-512.
The C5-propynyl-2'-OMe-CE Phosphoramidites have been discontinued.
Br-U-CE Phosphoramidite (10-3090)
5-I-U-CE Phosphoramidite (10-3091)