 |
|
|
|
 |
 |
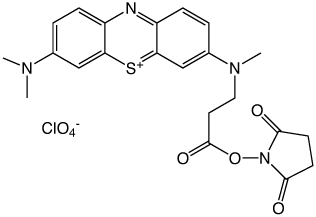
Methylene Blue NHS Ester (2) |
Methylene Blue II Phosphoramidite (3) |
Methylene Blue, which belongs to the phenothiazine family of dyes, is a unique dye with a variety of useful properties. Despite its high extinction coefficient in the visible region (81,000 L/mol.cm), it is weakly fluorescent due to its high rate of intersystem crossing from the S1 excited state to the T1 triplet state. This property makes it an excellent photosensitizer, and it has been used extensively to produce highly reactive singlet oxygen. In DNA, singlet oxygen leads to the oxidation of guanosine, resulting in the formation of 8-oxo-dG. Methylene Blue was subsequently used to determine that DNA polymerase eta (Pol η) was responsible for bypassing this lesion during replication.1
Another interesting property of methylene blue is its ability to both intercalate in duplex DNA, preferring G:C over T:A base pairs and its ability to act as an electrochemical redox probe.2 Exploiting this fact, Pheeny and Barton tethered methylene blue to a single-stranded probe attached to a gold surface to interrogate target oligonucleotides. They were able to detect a single mismatch in the probe-target duplex due to the reduced efficiency of charge transport through a DNA duplex containing a mismatch base pair.3
In a comprehensive study of redox-active reporters for electrochemical biosensors, it was found that methylene blue was unmatched in performance.4
In an earlier Glen Report,5 we introduced Methylene Blue C3 Phosphoramidite (1). Unfortunately, this product proved to have quite limited stability and has been discontinued. As an additional option, we introduced Methylene Blue NHS Ester (2) to allow researchers to label amino-modified oligonucleotides with this interesting dye.
With the encouragement and technical expertise of Carole Chaix and her colleagues at the University of Lyon, we decided to prepare an alternative structure that seemed to have a much superior stability profile - Methylene Blue II Phosphoramidite (3). Fortunately, this structure did indeed prove more stable and we are now able to offer again a Methylene Blue Phosphoramidite.
We recommend a 3 minute coupling for Methylene Blue II Phosphoramidite. In addition, UltraMild monomers and capping must be used to allow deprotection with 0.05M potassium carbonate in methanol (Catalog No. 60-4600-30).
The UV/Visible spectrum of an MB labelled oligo is shown in Figure 2.
We are happy to provide this unique electrochemical reporter, Methylene Blue II Phosphoramidite, in collaboration with Carole Chaix from the University of Lyon.
Methylene Blue II is covered under patent applications FR12 51739 and PCT/FR2013/050356 and is sold under license from the University of Lyon.
1. D.H. Lee, and G.P. Pfeifer, Mutat Res, 2008, 641, 19-26.
2. C.G. Pheeney, and J.K. Barton, Langmuir, 2012, 28, 7063-70.
3. E. Tuite, and B. Norden, J. Amer. Chem. Soc., 1994, 116, 7548-7556.
4. D. Kang, F. Ricci, R.J. White, and K.W. Plaxco, Anal Chem, 2016, 88, 10452-10458.
5. The Glen Report, 2013, 25.2, 1-2.
Methylene Blue NHS Ester (50-1960)
Methylene Blue II Phosphoramidite (10-5961)