In previous Glen Reports, we have presented Technical Briefs covering: "Which 5'-Amino-Modifier?"; and "Chemical Phosphorylation, Considering The Options". Therefore, we think it would be timely to present this article on "Which 3'-Amino-Modifier?"
We offer two types of 3'-amino-modifier – the first consists of a pair of branched chain linkers where the amine is protected with the ubiquitous fluorenylmethoxycarbonyl (Fmoc) protecting group; and the second uses a straight chain linker to the amine connecting to the support through a phthaloyl (PT) amide group.

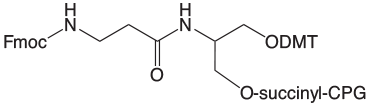
Our selection of Fmoc-protected 3'-Amino-Modifiers is shown in Figure 1. Both supports are based on a 1,3-diol backbone with a 6 atom linker to the Fmoc-protected amino group. We prefer the 1,3-diol type linkage to the support since it is very much less likely to eliminate on deprotection than the alternative 1,2-diol linkage. The mechanism of this elimination reaction is detailed in Figure 2. In contrast, the 1,3-diol does not have the same tendency to form the cyclic phosphate intermediate that leads to elimination of the linker to 3'-OH.

A consequence of the branch in the linker to accommodate attachment to both the support and the DMT group is a chiral center at the branch point. Once an oligo is synthesized, cleaved and deprotected, the chiral branch point leads to a pair of diastereomers, which can be separated chromatographically. However, the diastereomers are normally only observed in short oligos.
One of the major issues we have observed with Fmoc protection over the years is that the group can be replaced with acetyl during capping of the bulk support in the production process. This acetyl group of the protected amine impurity is not removed during regular oligonucleotide deprotection and so that percentage of available amine for further reaction is lost.
Interestingly, the percentage of the acetyl capped impurity is generally formed in a significantly higher amount in the production of 500Å CPG than 1000Å CPG. The average content of the acetyl capped impurity in the 500Å batches released over the last two years was 3.7%. In the case of the 1000Å CPG version, the average content was 1.6%.
The last two years of batches of 500Å and 1000Å CPG also revealed that the average loading of 500Å batches (20-2957) was 43.9 µmoles/g with a range of 38-49 µmoles/g. The dropoff point (where the synthesis begins to falter due to steric hindrance) has averaged 60-mer in length. The average loading of the equivalent 1000Å batches (20-2958) was 43.4 µmoles/g with a range of 36-48 µmoles/g. The average dropoff point was >100-mer in length.
The 500Å CPG version is a historical anomaly in our catalog in that we use 1000Å CPG for all of our other modifiers. The data provided in this article clearly show that the 1000Å CPG product is superior in quality, and consequently in performance, to the 500Å equivalent. Effective January 1, 2015, this support is being discontinued and we will routinely stock only the 1000Å version.
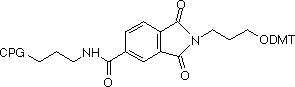
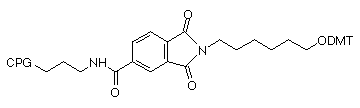
We offer two linker lengths in our 3'-PT-Amino-Modifiers, C3 and C6, and the structures are shown in Figure 1. In these supports, the amino group that is destined to be the 3'-amino-modification is incorporated into a phthaloyl (PT) group and is fully protected throughout the synthesis procedure. The amino group is then fully hydrolyzed from the phthaloyl moiety under conditions shown in Table 1. Although cleavage in ammonium hydroxide is fairly slow, it should be noted that standard cleavage/deprotection with AMA at 65°C for 10 minutes is sufficient for complete hydrolysis to the primary amine. There are no side reactions and only pure 3'-alkylamine is released into solution. In addition, there is no chiral center in the linker so no diastereomers with the potential to confuse future purification steps are formed on deprotection.
| Deprotection Reagent | Deprotection Conditions | Time |
|---|---|---|
| Ammonium Hydroxide | Room Temperature | 48 hours |
| Ammonium Hydroxide | 55°C | 17 hours |
| AMA | Room Temperature | 2 hours |
| AMA | 65°C | 10 minutes |
| 0.4M NaOH in methanol/water (4:1) | Room Temperature | 17 hours |
| 0.05M Potassium carbonate in methanol | Not Compatible | |
Our 3'-Amino-Modifiers are popular products but how do you choose which is appropriate for your application? Table 2 contains a comparison of these two types of 3'-Amino-Modifiers and demonstrates the pros and cons of both.
| Fmoc-Protected Amino Supports3'-PT-Amino-Modifiers | |
|---|---|
| Uses | Uses |
|
|
| Pros | Pros |
|
|
| Cons | Cons |
|
|
3'-Amino-Modifier C7 CPG (20-2958)
3'-Amino-Modifier Serinol CPG (20-2997)