Glen Research has a variety of options available for thiol-modification of the 3’ or 5’ terminus of oligonucleotides. However, the options available for thiol-modification within the sequence are much more limited. We do offer a complete range of amino-modified nucleoside phosphoramidites and oligonucleotides containing these can be modified using a hetero bifunctional crosslinker to convert the amino groups to thiols. The original crosslinker used for this purpose was Traut’s Reagent which simply converts an amino linkage to a thiol linkage. However, post-synthesis conjugation reactions are tricky to carry out and are sometimes not very efficient.
A better solution to this problem would be to modify an amino nucleoside, such as Amino-Modifier C6-dT, to a protected thiol prior to phosphoramidite production. Hence, we are pleased to introduce S-Bz-Thiol-Modifier C6-dT (1) to join the ranks of thiol-modifiers for oligonucleotide synthesis.
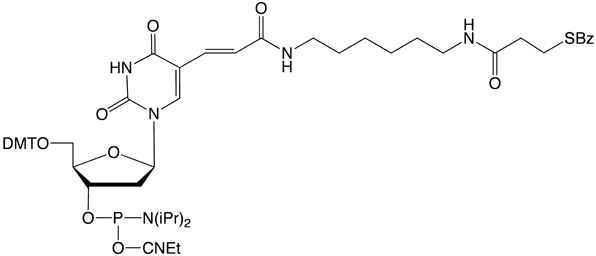
Thiol-Modifier C6-dT can be added as usual at the desired locations within a sequence. A coupling time of 3 minutes is recommended. No other changes from normal synthesis protocols are needed.
Prior to cleavage from the synthesis column, the support must be treated with 10% diethylamine (DEA) in acetonitrile to eliminate the cyanoethyl protecting groups from the phosphate backbone and to remove the acrylonitrile formed. This has to be done before cleavage and deprotection since the deprotected thiol would be very susceptible to alkylation by the acrylonitrile.
Cleavage and deprotection are then carried out using AMA for 2 hours at room temperature. Deprotection with ammonium hydroxide is not recommended for oligonucleotides containing Thiol-Modifier C6-dT since significant degradation is observed.
If oligonucleotides containing Thiol-Modifier C6-dT are to be used immediately for conjugation, 100mM tris(2-carboxyethyl)phosphine (TCEP) should be added to the AMA solution. Dithiothreitol (DTT) can also be used to keep the thiol in its reduced form but conjugation efficiencies are slightly higher (2-3%) if TCEP is used.
Oligonucleotides containing Thiol-Modifier C6-dT can be purified using RP cartridges, such as Glen-Pak™ cartridges, but the DMT group should not be removed on the cartridge with 2% TFA. Rather, the oligo should be eluted DMT-ON and the DMT group removed with aqueous acetic acid.
If oligonucleotides containing Thiol-Modifier C6-dT need to be stored prior to use, they should be reduced with 100mM TCEP in water to reverse any disulfide formation and then desalted into the conjugation buffer. The conjugation with an appropriate maleimide or iodoacetamide can then be carried out as normal.
S-Bz-Thiol-Modifier C6-dT (10-1538)
Glen-Pak™ is a trademark of Glen Research Corporation.