Albert Sánchez, Enrique Pedroso and Anna Grandas
Departament de Química Orgànica and IBUB
Facultat de Química, Universitat de Barcelona
Martí i Franquès 1-11
08028 Barcelona, Spain
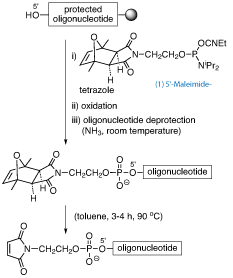
Two conjugation methodologies make use of maleimide groups, the maleimide-thiol Michael reaction and the Diels-Alder cycloaddition. Maleimides cannot be linked to the oligonucleotide while still anchored to the solid support because of their incompatibility with standard deprotection conditions, which make use of nucleophilic bases such as ammonia.
So far the most common alternative to prepare maleimido-oligonucleotides has been to react, in solution, bifunctional reagents incorporating a maleimide and a carboxyl group (or the corresponding active ester) with amino-derivatized oligonucleotides.1 However, reaction yields are not reproducibly high.
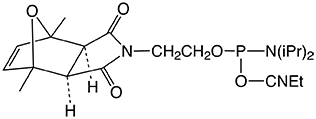
Maleimide protection provides a simpler, straightforward solution, since it allows the maleimide building block to be introduced on-resin into the protected oligonucleotide.2 For this purpose, phosphoramidite derivative (1) has been developed. This reagent incorporates a maleimide-2,5-dimethylfuran cycloadduct (exo isomer), which is stable to ammonium hydroxide at room temperature. This phosphoramidite (1) can be incorporated into DNA and RNA with both phosphate and phosphorothioate linkages.3
The oligonucleotide chain must be assembled using “ultra mild” phosphoramidites to avoid heating with ammonia. Then, following oligonucleotide elongation, the protected maleimide can be introduced at the 5’ end using phosphoramidite (1) and standard phosphite triester chemistry. Treatment with concentrated aqueous ammonia at room temperature removes protecting groups on the oligonucleotide chain, affording stable [protected maleimido]-oligonucleotides that can be purified as normal.
For the preparation of maleimido-oligoribonucleotides, deprotection of 2’-hydroxyl groups is carried out by reaction with Et3N·3HF (8 h, room temperature) before removal of the maleimide protecting group.
A retro–Diels-Alder reaction deprotects the maleimide. The [protected maleimido]-oligonucleotide is first dried by coevaporation with toluene (3x). Further addition of toluene (the amount that would be required to yield a 25 µM solution) provides a suspension that is heated in a metal block for 3-4 h at 90°C, and solvent is then removed in vacuo. The resulting residue is the target maleimido-oligonucleotide. Maleimide deprotection must be carried out immediately before use in conjugation reactions, because maleimido-oligonucleotides are not very stable.2
5'-Maleimide Modifier Phosphoramidite (1) is protected by a patent application and is offered by Glen Research under a non-exclusive license agreement from the University of Barcelona.