In 1997, Glen Research introduced our first Universal Support, popularly known as the McLean support, (1) in Figure 1.1 In principle, this support allows every oligonucleotide synthesis to take place on a single support without the need for a support for every desired 3’ nucleoside. The support found favor in high-throughput oligonucleotide synthesis platforms and became the universal support of choice for deprotection using gaseous methylamine. However, since the phosphate elimination (dephosphorylation) reaction to yield a free 3’-hydroxyl group requires quite aggressive conditions, as outlined in Table 1, this product has been somewhat limited in its applications. The main problem was the fact that the product could not be used to prepare oligos with some of our most popular nucleoside analogues at the 3’ terminus since many of these are not compatible with the required aggressive dephosphorylation conditions. Hence, we felt it was time to investigate other supports to determine if an alternative would offer better overall properties.



| Reagent | Conditions |
|---|---|
| Ammonium Hydroxide | 80°C/8h min. |
| Ammonium Hydroxide/ 40% Methylamine (AMA) | 55°C/17h |
| 80°C/3h min. | |
| 40% Methylamine | 55°C/8h |
| 0.4M NaOH in Methanol/Water (4:1) | 80°C/0.5h |
| 55°C/3h | |
| RT/17h |
| Reagent | Conditions |
|---|---|
| Ammonium Hydroxide | 80°C/2h |
| 55°C/8h | |
| Ammonium Hydroxide/ 40% Methylamine (AMA) | 80°C/0.5h |
| 65°C/1h | |
| 55°C/2h | |
| Methylamine Gas | 65°C/30min at 30 psi |
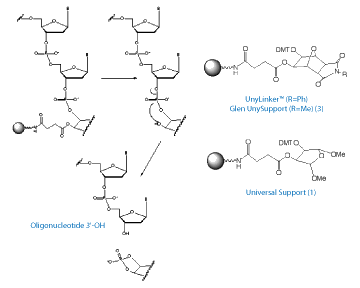
Our original Universal Support (1) is one of a family of universal supports where the cleavage from the support is followed by deprotection and dephosphorylation.2 Usually in this type of support (Type 1), dephosphorylation is the slowest step. The process is illustrated in Figure 2.
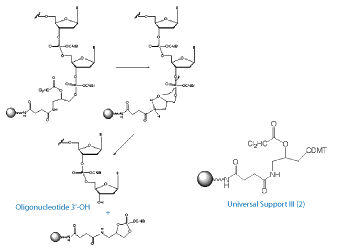
Glen Research’s work on universal supports has culminated in the development of Universal Support III, (2) in Figure 1. US III is unique among universal supports in that the dephosphorylation reaction occurs first and leads to cleavage from the support (Type 2).2 This reaction occurs with mild anhydrous methanolic ammonia, or even with the UltraMild deprotection solution of anhydrous potassium carbonate in methanol. Oligonucleotide deprotection can then be carried out using your favorite procedure - AMA, ammonium hydroxide, and all other common methods. The process is illustrated in Figure 3, Page 11.
Why is there a need for any universal support other than our US III? After all, US III is truly universal in that it can be used for ANY oligonucleotide synthesis, from DNA with very sensitive bases, through siRNA, to exceptionally sensitive labelled probes. However, the one minor issue with US III is that it is not readily compatible with gas phase cleavage and deprotection using anhydrous methylamine gas, and this strategy is used in many high-throughput situations. It appears that the elimination of cyanoethyl protecting groups is favored in anhydrous methylamine gas. Once the cyanoethyl protecting group is eliminated to give the phosphodiester group, the amide-assisted dephosphorylation reaction to give the desired 3’-OH grinds to a halt. The yield of isolated oligonucleotide therefore suffers.
Our original Universal Support (1) is compatible with deprotection using anhydrous methylamine gas and has been used extensively, but the time for complete dephosphorylation is longer than most companies wish to allocate. A recent development has been the use of a support based on a molecule which is “conformationally preorganized” to accelerate the dephosphorylation reaction.3,4 By using a rigid bicyclic molecule on the support (3) in Figure 1, Page 10, it was hoped that the molecule's conformation would facilitate the formation of the cyclic phosphate transition state, thereby stimulating the rate of dephosphorylation, as shown in Figure 2, Page 10. And, indeed, it was found that the rate of elimination is markedly faster than the original Universal Support (1), as shown in Table 2, Page 10.
The structure of Glen UnySupport is shown, (3) in Figure 1, Page 10. The phenyl version, developed at Isis Pharmaceuticals as UnyLinker™, is available from several companies for large scale oligo synthesis. Glen UnySupport is the methyl version, which is preferred for high throughput oligonucleotide synthesis since methylamine rather than aniline is formed on deprotection. We are happy to offer Glen UnySupport in a variety of popular formats under license from Isis Pharmaceuticals.
This product is covered by US Patent 7,202,264 owned by Isis Pharmaceuticals, Inc..