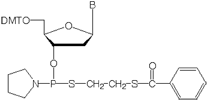
Thiophosphoramidites are activated modified deoxyribonucleotides which effect the substitution of both internucleotide nonbridging oxygen atoms with sulfur. The resultant oligo has an achiral internucleotide phosphorodithioate (PS2) linkage. The structure of a thiophosphoramidite is shown in Figure 1.
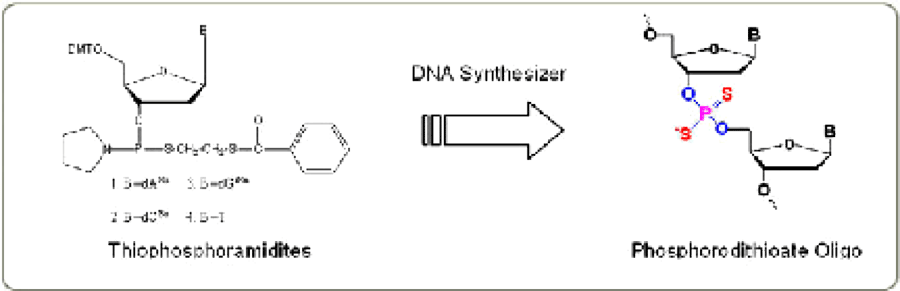
An achiral dithio linkage is produced by the coupling of the appropriate thiophosphoramidite and the subsequent sulfurization step using Beaucage Reagent. The reaction is shown in Figure 2.
Initially, PS2-oligos were investigated as potential antisense compounds and exhibited the ability to interfere with the expression of erbB-2 mRNA associated with breast cancer, to inhibit HIV-1 reverse transcription activity, and to induce B-cell proliferation and differentiation.1,2 The HIV-1 inhibition of HIV-1 reverse transcriptase is dependent on the number of dithioate linkages and the length of the dithioate oligo.1,3 A comparative analysis with phosphoromonothioate equivalents indicates that dithioate oligos are much better inhibitors and are able to inhibit potently with relatively short oligomer length.1,3 Inhibition of HIV-1 reverse transcriptase by PS2-oligos appears to be a general phenomenon as all of the nucleotide base sequences examined inhibit its activity.
What are the attributes and applications for phosphorodithioate oligonucleotides (PS2-oligos)?
PS2-oligos have potentially useful characteristics such as: 1) high binding affinities to proteins and cell surfaces; 2) are nuclease resistant and therefore stable in biological preparations; 3) are easily prepared; and 4) as thioaptamers show excellent specificity to proteins.4 Significantly, the PS2-oligos, in contrast to the monothiophosphate oligos (S-oligos), are achiral about the dithiophosphate center, so problems associated with diastereomers are completely avoided.
Oligos with high proportions of phosphorothio and dithio linkages appear to lose some of their specificity and are “stickier” toward proteins in general than oligos with normal phosphate esters, an effect often attributed to non-specific interactions. This can be quite important since the recognition of nucleic acid sequences by proteins involves specific side chain and backbone interactions with both the nucleic acid bases as well as the phosphate ester backbone. One can take advantage of this “stickiness” to enhance the affinity of PS2-oligos for a protein target but the total number of PS2 linkages must be optimized to decrease non-specific binding to the protein target and only enhance the specific favorable interactions with the specific proteins.
PS2 analogues have been successfully used as aptamers for a variety of protein targets including activated protein 1 (AP-1) as well as transcription factor NF-κB for which the PS2-oligos demonstrated 150 picomolar Kd with a dissociation time >12 hours. PS2-oligos demonstrate up to 300x greater binding affinity for proteins than oligos without PS2 linkages with no loss of aptamer specificity. For HIV-1 RT, dithioates bind 28 (vs. monothioate) or 600 (normal backbone)?times more tightly than the normal aptamer oligonucleotide or the S-analogue. For aptamer applications, it is recommended that no more than six dithio linkages be included in any PS2-oligo in order to minimize non-specific protein interactions.
PS2 oligos have been used in a variety of assays, instruments and technologies offering a wide field of use. They have shown utility in gel shift assays, nanoparticle technologies, microarrays, BioPlex based applications as well as in diagnostic applications.5,6
The Tm of PS2-oligos is lower than the equivalent PS-oligo or the normal phosphate ester, with the degree of Tm depression paralleling the percent phosphorodithioate composition of the oligomer. It is in the range of 0.5 ~ 1.5 °C per dithio linkage.
Toxicity has not been observed in cell culture assays with PS2-oligos.1,3
Phosphorodithioate and phosphoro-monothioate anions preferentially bind to Cd2+ and Mn2+ while phosphate ester anions preferentially bind to Mg2+ which may provide a mechanism to demonstrate protein binding domains for specific oligo sequences.7,8
Thiophosphoramidites can be used on a variety of commercial synthesizers with only slight changes in protocol.
Extended coupling times and sulfurization times are recommended to ensure optimum coupling efficiency and complete sulfurization. Coupling times of six minutes and sulfurization times of six minutes have been used successfully with the Expedite synthesizer. Caution: if sulfurization is incomplete, a proportion of PS linkages will be present along with dithio linkages.
Monomer concentrations are typically 0.05M or higher. Monomers are dissolved in anhydrous acetonitrile with a small proportion of dichloromethane. Typical coupling efficiencies exceed 90%. The monomers have a lifetime of two days on the synthesizer.
Cleavage conditions for PS2-oligos are equivalent to normal phosphate ester oligos and are amenable to various known cleavage cocktails and conditions.9-11
PS2-oligos have been purified by PAGE, RP-HPLC, and IEX-HPLC.11-13
IEX-HPLC at pH 8 is the preferred method. Thiocyanate (SCN-) as the eluting ion can be used for oligos with a high proportion of PS2 linkages. PS2-oligos are separated on the basis of charge and the number of sulfur substitutions. Purities of oligos (2-30 mers) of greater than 95% are routinely achieved. Highly purified PS2-oligos with less than 50% PS2 linkages have been obtained. The retention times of PS2-oligos on a MonoQ column are in direct proportion to the number of PS2 linkages. The average retention time increase is 2.3 minutes per sulfur addition on MonoQ 5/5 columns.14
PAGE allows for excellent pure preparations but the technique suffers from manual gel excision and typically low yields.
RP-HPLC is used with the DMT-on method but is not a method of choice because of the hydrophobicity of the PS2-oligo. In addition, it can be difficult to remove the DMT-on PS-oligo impurities (de-sulfuration products) using RP-HPLC. Crude PS2-oligo chromatograms are routinely complex and identifying the full length oligo is not always obvious with UV detection.
PS2-oligos can be confirmed by MALDI-TOF MS and 31P-NMR. Resonances of PS2 linkage (112 ppm), PS linkage (58 ppm), phosphate (0 ppm) in 31P-NMR spectra are well resolved.15,16
The thiomonomers are patented (U.S. patent 5,218,088) by and sold under an agreement with AM Biotechnologies. Products are for research purposes only. Products may not be used for diagnostic, clinical, commercial or other use, including use in humans.
The thiomonomers are patented (U.S. patent 5,218,088) by and sold under an agreement with AM Biotechnologies. Products are for research purposes only. Products may not be used for diagnostic, clinical, commercial or other use, including use in humans.